WHO names Coldrif among 3 'substandard' cough syrups in India
What's the story
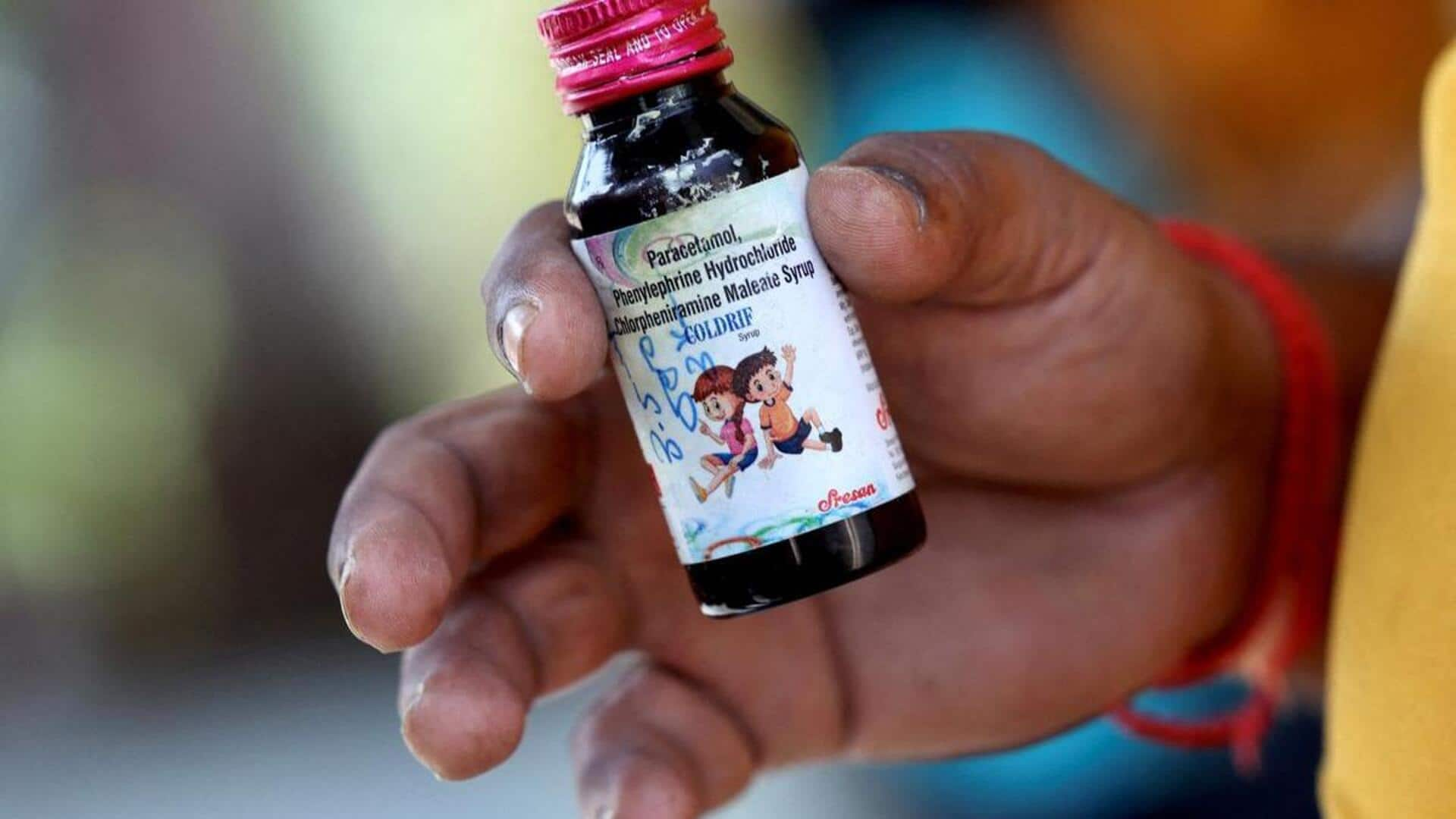
The World Health Organization (WHO) has issued an alert on three "substandard" cough syrups in India, including Coldrif, Respifresh TR, and ReLife. The warning comes after the deaths of 22 children in Madhya Pradesh, mostly under five years old, due to suspected kidney failure after consuming Coldrif. The WHO has urged global regulatory authorities to report any findings of these products immediately.
Toxicity revealed
DEG contamination in oral liquid medications
The WHO alert specifically mentioned the presence of Diethylene Glycol (DEG), a toxic substance, in at least three oral liquid medications. The Central Drugs Standard Control Organisation (CDSCO) of India had informed the WHO on October 8 about this contamination. The CDSCO clarified that affected children had consumed these products, and state authorities have since stopped production at manufacturing sites and suspended product authorizations.
Surveillance advised
Recommendations for National Regulatory Authorities (NRAs)
The WHO has also recommended that National Regulatory Authorities (NRAs) consider targeted market surveillance, especially in informal and unregulated supply chains. It has advised NRAs to assess the risks of any oral liquid medicines from the same manufacturing sites since December 2024. The toxic effects of DEG include abdominal pain, vomiting, diarrhea, headache, altered mental status, and acute kidney injury, which can be fatal.
Authorities' action
Action taken by Indian authorities
In light of the deaths, state authorities have taken immediate action. Sresan Pharmaceuticals, the manufacturer of Coldrif, had its manufacturing license revoked. Subsequently, its owner was also arrested. The government has also issued an advisory cautioning against prescribing cough syrups to children under two years old, and generally not recommended for those under five.