First malaria drug for newborns, young children approved for use
What's the story
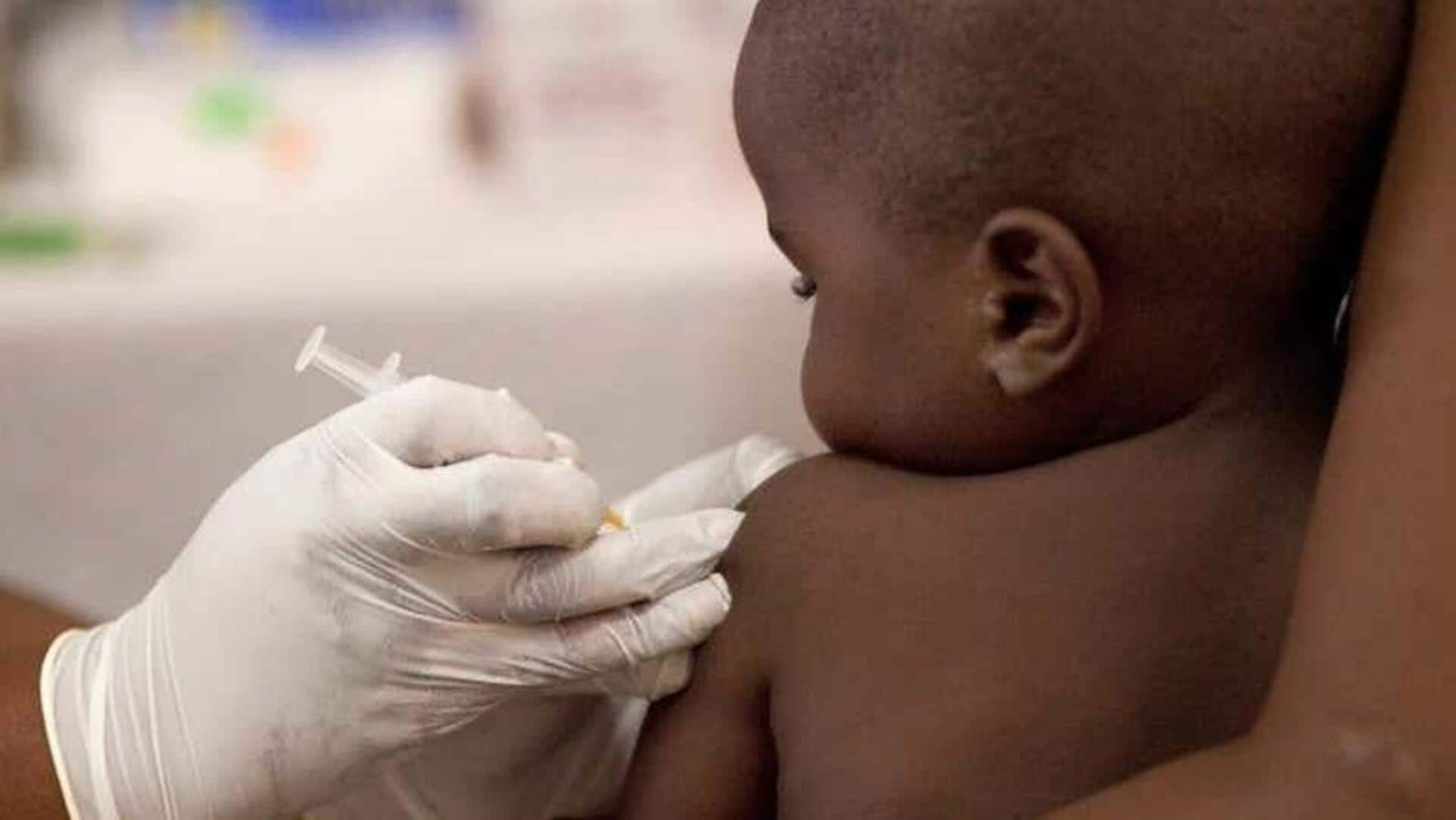
The first-ever malaria treatment specifically designed for babies and young children has been approved for use. The drug, developed by Swiss pharmaceutical giant Novartis, will soon be available in African countries, where the disease is most prevalent. Until now, babies were treated with formulations for older children of malaria drugs, which posed a risk of overdose due to their different body weights and liver functions.
Child mortality
Malaria kills nearly 600,000 people every year
In 2023, malaria was responsible for some 597,000 deaths globally. Africa bore the brunt of this burden, with three-quarters of the fatalities being children under five years old. The new drug from Novartis seeks to fill a major "treatment gap" in this vulnerable population by providing a safe and effective treatment specifically tailored for babies and young children weighing less than 4.5kg.
Corporate responsibility
Coartem Baby developed in partnership with MMV
Vas Narasimhan, CEO of Novartis, emphasized the company's long-standing dedication to fighting malaria. He said, "For more than three decades, we have stayed the course in the fight against malaria." The new drug—Coartem Baby or Riamet Baby in some countries—was developed by Novartis in partnership with Medicines for Malaria Venture (MMV), a Swiss-based non-profit organization. Eight African nations participated in its evaluation and trials.
Malaria elimination
Treatment will save countless lives, say experts
Martin Fitchet, CEO of MMV, hailed the approval of Coartem Baby as a major step toward eliminating malaria. He said, "Malaria is one of the world's deadliest diseases, particularly among children. But with the right resources and focus, it can be eliminated." Dr. Marvelle Brown from University of Hertfordshire also stressed on this breakthrough in saving lives of babies and young children suffering from malarial infections.