India approves Mounjaro KwikPen for diabetes and obesity treatment
What's the story
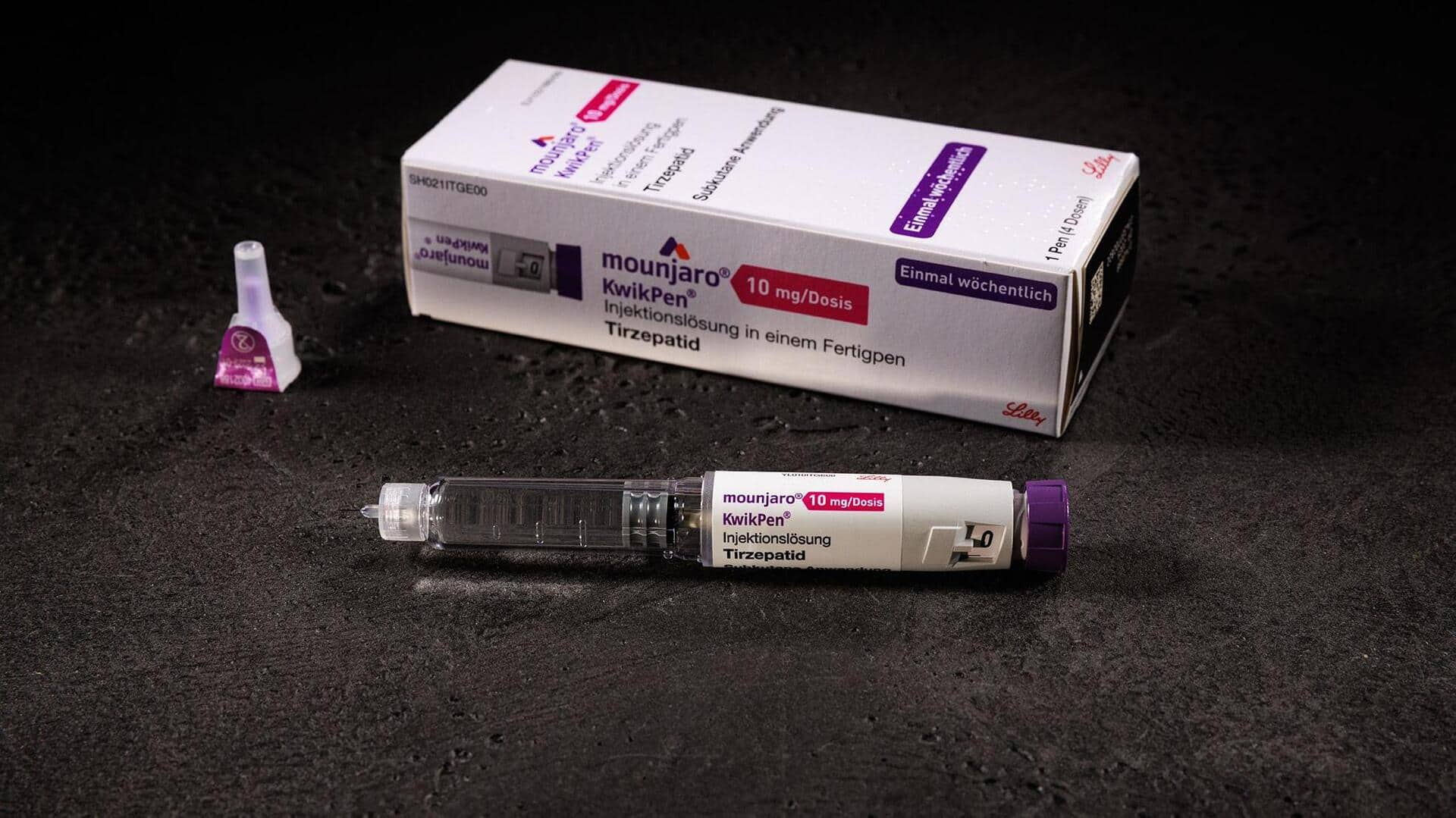
Eli Lilly has received regulatory approval from the Central Drugs Standard Control Organization (CDSCO) for its diabetes and weight-loss drug, Mounjaro. The approval is specifically for the KwikPen device, which will be used to administer the medication. This comes nearly three months after Mounjaro was first launched in India in vial form.
Drug details
Mounjaro: A first-of-its-kind medication
Mounjaro (tirzepatide) is a first-of-its-kind medication that works as a dual GIP (glucose-dependent insulinotropic polypeptide) and GLP-1 (glucagon-like peptide-1) receptor agonist. It is prescribed for Type 2 diabetes and chronic weight management in adults with obesity. The KwikPen device is a multi-dose, single-patient-use pre-filled pen designed for once-weekly administration of the drug.
Treatment approach
Approval of this device is a major step
Each Mounjaro KwikPen delivers four fixed doses of 0.6ml and has been approved in six dose strengths: 2.5mg, 5mg, 7.5mg, 10mg, 12.5mg, and 15mg. Winselow Tucker, President and General Manager of Lilly India said that the approval of this device is a major step in treating people with Type 2 diabetes and obesity. He also emphasized how it will enable healthcare professionals to customize care according to individual patient needs and clinical goals.